Here’s a great video tip from LabTricks.
Please note that this video is unaffilated with Bio-Rad Laboratories.
Here’s a great video tip from LabTricks.
Please note that this video is unaffilated with Bio-Rad Laboratories.
Courtesy of LabTricks.
Are your glass plates getting old? Are chips and cracks causing your gels to leak? Checkout this great tip from Benchfly on how to prevent leaks when pouring plates for SDS-PAGE.
Have any other suggestions? Please share!
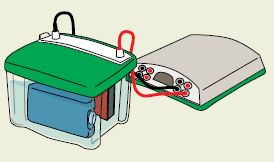 Power supplies that are used for electrophoresis hold one parameter constant (either voltage, current, or power). The PowerPac™ HC and PowerPac Universal power supplies also have an automatic crossover capability that allows the power supply to switch over to a variable parameter if a set output limit is reached. This helps prevent damage to the transfer cell.
Power supplies that are used for electrophoresis hold one parameter constant (either voltage, current, or power). The PowerPac™ HC and PowerPac Universal power supplies also have an automatic crossover capability that allows the power supply to switch over to a variable parameter if a set output limit is reached. This helps prevent damage to the transfer cell.
During transfer, if the resistance in the system decreases as a result of Joule heating, the consequences are different and depend on which parameter is held constant.
Transfers Under Constant Voltage
If the voltage is held constant throughout a transfer, the current in most transfer systems increases as the resistance drops due to heating (the exception is most semi-dry systems, where current actually drops as a result of buffer depletion). Therefore, the overall power increases during transfer, and more heating occurs. Despite the increased risk of heating, a constant voltage ensures that field strength remains constant, providing the most efficient transfer possible for tank blotting methods. Use of the cooling elements available with the various tank blotting systems should prevent problems with heating.
Transfers Under Constant Current
If the current is held constant during a run, a decrease in resistance results in a decrease in voltage and power over time. Though heating is minimized, proteins are transferred more slowly due to decreased field strength.
Transfers Under Constant Power
If the power is held constant during a transfer, changes in resistance result in increases in current, but to a lesser degree than when voltage is held constant. Constant power is an alternative to constant current for regulating heat production during transfer.
The above information was adapted from Bio-Rad’s protein blotting guide. For more great information, be sure to download the Protein Blotting Guide from Bio-Rad Laboratories.
 The following are the top 8 causes for overall high background on your western blot and potential solutions:
The following are the top 8 causes for overall high background on your western blot and potential solutions:
For more great information, download the protein blotting guide from Bio-Rad Laboratories.