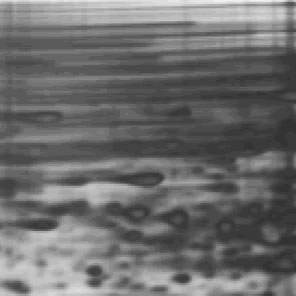
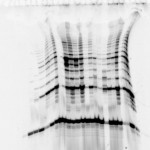
Last week, at the request of our friend Adriana from the University of Toronto, we posted an ugly gel mystery which she needed help solving. Thanks to our educated readership, we were blessed with a multitude of answers. Behold the power of social networks!
The answers included comments from….
Nicole Harris who said “It looks to me like the gel did not set smoothly. Possiable knocked while setting or started to set prior to pouring, causing ripples to form.”
Kirk who said “There maybe within the gel a electro magnetic polarization of molecules responding to natural magnetic fields that developed while curing. A molecular analysis studying the differentiation between the dark and light areas.”
And Dan Moran “Looks like a protein overload first. Then it looks like it was terribly over heated due to the use of the wrong buffer in the wrong gel resulting in high resistance, boiling buffer and melting gel.”
Now here’s an answer from our friendly technical support reps, David Palmer and Cecilia Carino of Bio-Rad Laboratories who gave Andrea the following detailed response:
1)Some sequencing users (yes…this is a sequencing gel folks! ed insertion) apply an excessive amount of power during the pre run to achieve rapid warming of the gel. More heat is generated than can be efficiently distributed by the system under these circumstances. A better (and faster) way to warm up the gel would be to preheat the buffer in a microwave before adding to the IPC. Half the buffer can be heated to almost boiling and then added to the other half at room temperature resulting in a temperature very near the desired 50-55 degrees. Samples can be loaded immediately in this protocol.
2) If the gel reagents are old, have been incompletely mixed, or were not degassed prior to pouring there could be incomplete polymerization along the sides. This in turn could affect the flow of electrical current such that an inordinate amount of heating occurs in close proximity to the spacers. It might be that the first gels you run were cast with fresh reagents, and over time, the reagents have became old. Check and make new reagents if needed.
So….check your reagents people. Old reagents that have been sitting on the shelf for months (you know who you are) should be replaced. Making buffers is a pain, but it’s a much bigger pain to run you precious samples in a gel only to find out that everything has been lost!
As you can see, there’s a real power in “turning to the masses.” Keep sending in your ugly gels and we will do whatever we can to help you solve your ugly mystery!
Stay tuned for another ugly gel to be posted later this week.