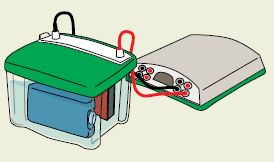
All gels are not created equal when it comes to protein transfer. Mini-PROTEAN® TGX™ precast gels, together with the Trans-Blot® Turbo™ transfer system, offer you a complete solution for achieving fast electrophoresis and transfer times. In addition to speed, these innovative gels deliver maximum transfer of proteins onto membranes so you don’t see proteins left behind on the gel, ensuring quality western blotting results you can count on every time.
For more information visit Bio-Rad Laboratories TGX™ precast gel page.